Patients have been asking me about a new biostimulator called GOURI — a injectable treatment which contains “Liquid PCL (Polycaprolactone) “. Some clinics locally and overseas have been offering it, with some doctors in Singapore actively promoting it. The treatment promises to rejuvenate the skin through collagen stimulation. The treatment is exciting and promising. However, I am very careful when it comes to new products, and hence will exercise caution before using them on my patients.
GOURI: From the Manufacturer
From the manufacturer: “GOURI uses a patented technology which enables regeneration of natural skin collagen and rejuvenates your skin without the use of microparticles. It is totally different from other high polymer products. Existing products are not fully liquid. They are formed by mixing of high polymer material powder (such as PCL and PLLA) with liquid saline or CMC gel. As a result, they can’t spread out into the entire face and have a high risk of complications. They give effect on the localized part only. Moreover, there is a risk of hypercorrection in cases when too much of material is injected. GOURI as the 1st fully liquid type PCL injectable, spreads out into the entire face.”
The treatment schedule of GOURI is not disimilar to many of the skinboosting and rejuvenating treatments out there. 3 sessions are recommended at 4-week intervals. After 3rd session, additional 1 or 2 sessions can be applied every 3-6 months to maintain the results.
GOURI: The Science
There has been some publications about GOURI. One study evaluated the effects of GOURIon the skin of rats1 . Rats were divided into 2 groups – one was injected with phosphate-buffered saline and the other with GOURI.
The positives: Biopsies showed did not show the presence of injected materials within the tissue samples, or any filler-induced foreign body reactions. Rats injected with GOURI showed a marked increase in dermal thickness, especially during the first 6 weeks. Skin elasticity was also increased. The study concluded that study GOURI particle-free PCL suspension can effectively induce neocollagenesis that leads to dermal rejuvenation1.
Another study compared the safety and efficacy of GOURI in correcting crow’s feet 2– the wrinkles at the sides of the eyes, vs Rejuran, an injectable polynucleotide treatment. In this split face study, 30 patients were randomised into either the GOURI or Rejuran group, and treated either on the left of right with GOURI, and treated with Rejuran on the other side. The improvements in wrinkle severity was then evaluated. Both patient and evaluator were blinded as to which product was being used on the left or the right.
The positives: The study showed that both GOURI and Rejuran were well tolerated. While the severity of Crow’s feet improved on both sides, the side treated with GOURI showed better improvement rates in the crow’s feet, though there was no statistical significance. The study concludes that that the novel DLMR01 (GOURI) is safe and performs similarly to RJR over 12 weeks2.
Available PCL Based Injectables

There is an existing PCL based injectable product available in the market – Ellanse. In contrast to GOURI, Ellanse contains PCL in the form of microspheres, suspended in a CMC (carboxymethyl cellulose) gel carrier. Ellanse is used as a filler giving volume replacement, with the additional benefit of skin rejuvenation from neocollagenesis.
Ellanse has been studied more extensively, and used in more countries, with an excellent track record. The adverse events related to Ellanse are very similar to Hyaluronic acid fillers. Most of them are temporary, such as swelling, bruising and redness. More serious side effects such as vascular occlusion can occur – the incidence is similar to that of Hyaluronic acid fillers, and can be mitigated with proper technique, slow and small volume injections, and adequate knowledge of anatomy. Delayed onset nodules and granulomas have also been reported5, but these are not common.
GOURI is said to have an advantage over Ellanse as there is less swelling and redness due to its liquid nature. It also claims to have more homogenous distribution all over the face to improve skin quality. It also cites ease of injection as an advantage, as liquid PCL do not aggregate and block thin needles, unlike in Ellanse. However, these advantages are, in my opinion, minor at best. Ellanse has minimal swelling and redness post treatment, and I personally do not have problems with needle blockage. I also prefer a filler where I can control where I am injecting and know where the particles are being deposited into the skin.
GOURI: Initial Questions
Where Are We Placing the Product?
When I first studied GOURI, it was indeed an exciting new rejuvenating treatment to offer our patients. However, I had some concerns and questions. In the study on rats, authors report that there was no evidence of any injected material in biopsy specimens. 3 dimensional assessment also showed that the injected material is almost completely absorbed within 6 hours1 . where does the injected material go then? Although PCL is a proven and very safe material, it is a foreign body in the skin, and we want to know where we are placing the material.
How is PCL Solubilised?

Polycaprolactone (PCL) is a biodegradable polyester with a low melting point of around 60 degrees celsius. PCL can be readily degraded by lipases and esterases of the microorganism3. It is biocompatible and nontoxic, and often found in the form of beads for industrial uses, or microspheres for medical use.
According to literature and to the manufacturer, “GOURI is manufactured by the Newly developed Collagenesis-Enabled Solubilized Active Biodegradable Polymer (CESABP) technology by DEXLEVO Inc, which enables the particle-free liquid type PCL filler formula. It permitted the homogenous distribution of PCL in water, thereby enhancing uniform tissue rejuvenation1.”
However, we know that PCL is hydrophobic material4, which means that it repels water and is NOT soluble in water, and instead soluble in nonpolar solvents such as benzene. Hence, from my limited knowledge, it is not possible to have a liquid PCL solution. I would love to find out more about CESABP technology and how solubility is achieved.
What is the Mechanism of Action?
The same paper first claims the superiority of liquid PCL, then quotes that “PCL micro- spheres seemed to act as nuclei for new collagen formation.” It seemed like the authors were not sure of how the skin rejuvenation and neocollagenesis came about after GOURI injections. This was an alarm bell for me. When I questioned further, I could not also find the mechanism of collagen stimulation anywhere. It was almost like – magic. and we know that certainly does not happen in medical aesthetics. It is all science, and sometimes. art.
How Long Does GOURI Stay in the Body?
We know that Ellanse microspheres stay in the injected areas for 1,2,3 or 4 years, depending on the microsphere composition. However, there is no way to tell for GOURI histologically as the PCL cannot be found anymore after treatment.
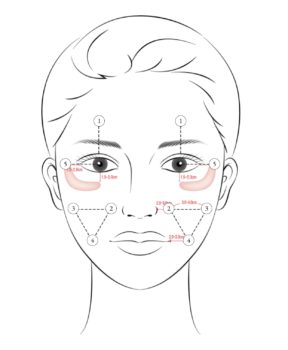
What is the 5 Point Injection Technique based On?
Following Profhilo’s 5 point injection technique, other treatments have also begun to advocate a similar technique. GOURI is one of them, with 5 points which are simple to administer. The 5 points cover both the upper and lower face. However, I am concerned about some of the 5 points.
Point 1, in the forehead, is in an area where there are many blood vessels traverse in the subcutaneous plane. For inexperienced injectors, inadvertent injection into the veins or artery can occur here.
Point 4 is in region of the superior and inferior jowl fat pads. Theoretically, injecting a product in a patient with existing, sagging jowls may worsen they, especially over repeated treatments. This has not been reported, but just a personal concern of mine.
The scientific papers treated the crow’s feet, but there is nothing described about the 5 points – what they are based on, their safety, and efficacy. Hence, I do wonder how they are derived.
GOURI: Emerging Concerns From Users and Patients
Due to the initial concerns, I never started offering the treatment to my patients, wanting to listen to the feedback of fellow colleagues before I decide to do so. Doctors who have performed the treatment have indeed report that patients’ skin quality improved, with improvement in skin tone, and pores especially.
However, along with the glowing (pardon the pun) reviews, doctors have also shared their anecdotal experiences about some adverse effects they came across, some minor, and others, more concerning, such as:
1. Prolonged bumps at injection sites.
Doctors and patients are experiencing nodules at injection sites – which can last for days, weeks or months. This may mean that GOURI is not as spreadable as it claims to be.
2. Prolonged Bruising
Patients have been reporting prolonged discoloration after GOURI, especially in the injection point in the malar area. The bruise is purplish, and persists up to 9 months after injection. I have personally come across 2 of such cases.
3. Allergic Reactions – Local and systemic
Some patients developed local allergic reactions after the treatment, in the form of swelling and redness around the injection site, or for some, more generalised reactions in the form of skin rashes. It is not clear if more severe forms of allergy, such as anaphylaxis, is a concern. Some doctors are now premedicating with antihistamines before GOURI treatment.
4. Fainting Spells and Obtunded Senses
Arguably, the most unexpected adverse effect is the report of patients developing temporary syncopy (fainting), and reporting that they feel that their senses are blunted immediately post treatment. It is not clear what causes this, and the effects seem temporary and resolve by themselves.
I Always Proceed Slowly With New Products
GOURI is an exciting new injectable treatment which rejuvenates the skin through collagen stimulation with liquid PCL.
As medical practitioners, we are often excited when a new product hits the market. However, it is also our responsibility to ensure that a product is safe and effective, and that we know everything about the product, before we offer it to our patients. In the case of GOURI, it is my personal preference to wait for more experience and data from scientists, doctors and patients, especially about the possible effects, before I can confidently offer this treatment for my patients.
In the meantime, I will prefer to use other alternatives for skin quality improvements, such as hyaluronic acid skinboosters, polynucleotides (PN), or hybrid cooperative complex (HCC) Hyaluronic acid. For a PCL based product, I often use Ellanse, for volumization and skin rejuvenation.
I would also love to gather feedback from fellow doctors, and patients who have undergone this treatment, so that we can better advise our patients.
References:
- Hong JY, Kim JH, Kwon TR, Hong JK, Li K, Kim BJ. In vivo evaluation of novel particle-free polycaprolactone fillers for safety, degradation, and neocollagenesis in a rat model. Dermatol Ther. 2021 Mar;34(2):e14770. doi: 10.1111/dth.14770. Epub 2021 Jan 18. PMID: 33421287.
- Jeong GJ, Ahn GR, Park SJ, Hong JY, Kim BJ. A randomized, patient/evaluator-blinded, split-face study to compare the efficacy and safety of polycaprolactone and polynucleotide fillers in the correction of crow’s feet: The latest biostimulatory dermal filler for crow’s feet. J Cosmet Dermatol. 2020 Jul;19(7):1593-1599. doi: 10.1111/jocd.13199. Epub 2019 Nov 4. PMID: 31680395.
- Current Developments in Biotechnology and Bioengineering. Chapter 32 – Biodegradation of Biopolymers N.R.Nair, V.C.Sekhar, K.M.Nampoothiri, A.Pandey
- Nanocomposites for Musculoskeletal Tissue Regeneration. Chapter 1 – Design and fabrication of nanocomposites for musculoskeletal tissue regeneration. N.Narayanan, L.Kuang, M.Del Ponte, C.Chain, M.Deng
- Chiang CH, Peng JH, Peng HP. Filler-induced granuloma from polycaprolactone-based collagen stimulator injection in the tear trough area: A case report. J Cosmet Dermatol. 2021 May;20(5):1529-1531. doi: 10.1111/jocd.14010. Epub 2021 Feb 26. PMID: 33600081.